 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
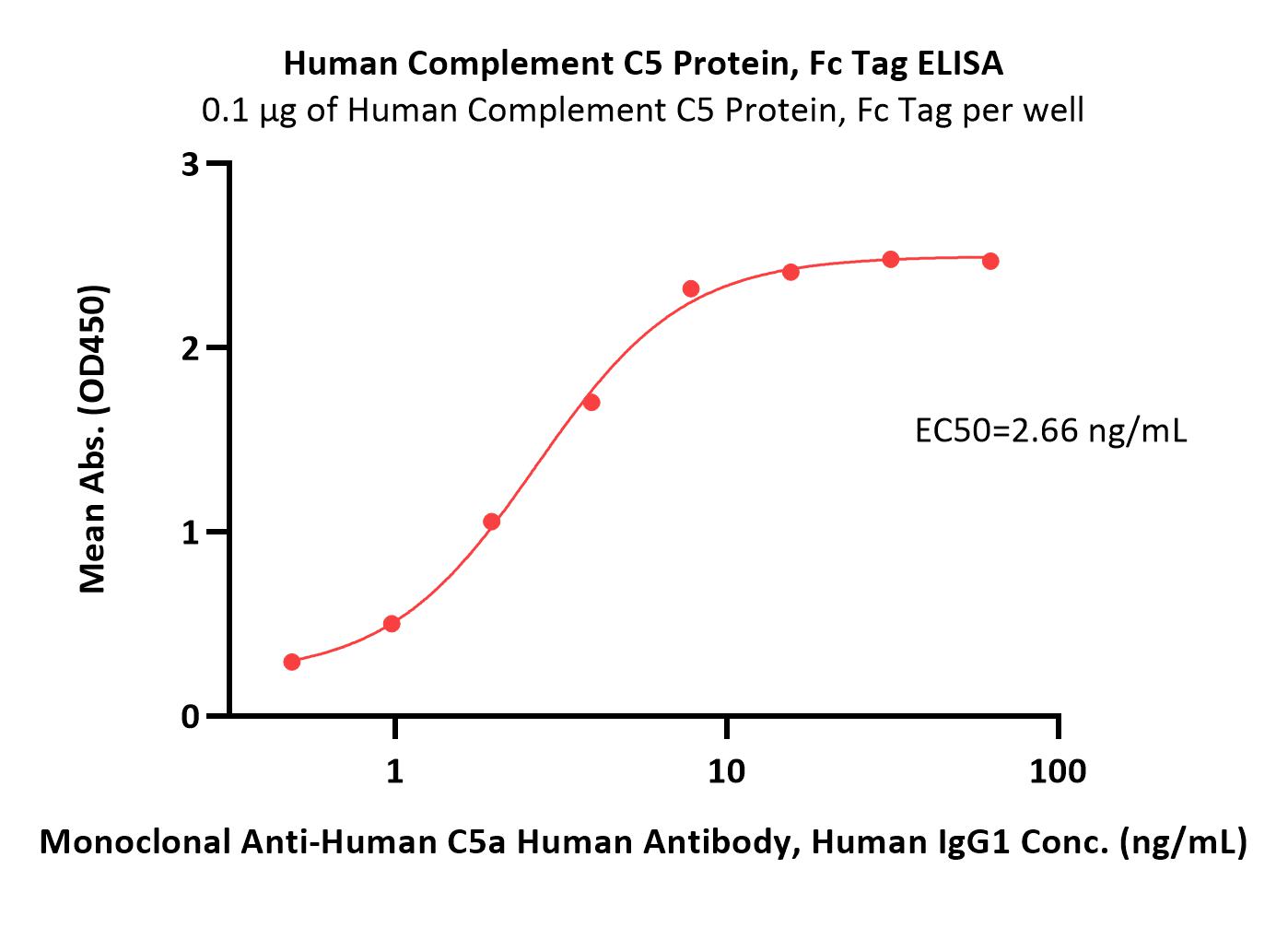
Immobilized Human Complement C5 Protein, Fc Tag (Cat. No. CO5-H5253) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Human C5a Human Antibody, Human IgG1 with a linear range of 0.5-4 ng/mL (QC tested).
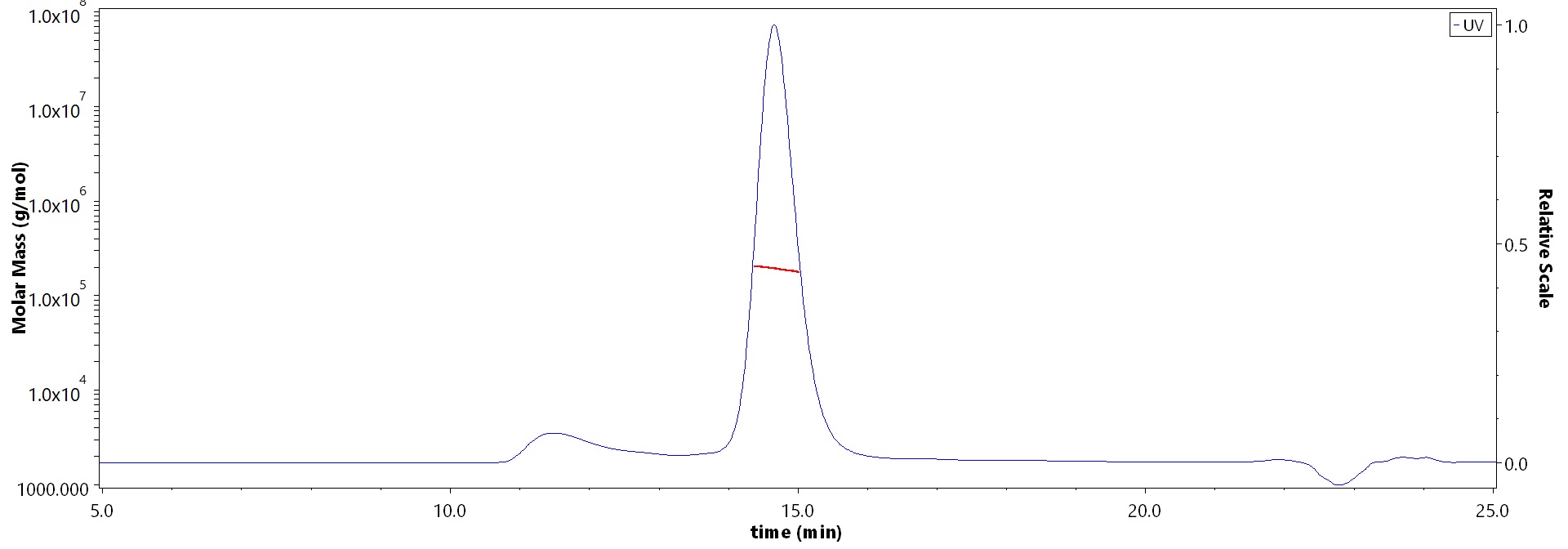
The purity of Biotinylated Cynomolgus Complement C5 Protein, His,Avitag (Cat. No. CO5-C82E3) is more than 85% and the molecular weight of this protein is around 170-210 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Avacincaptad pegol | ARC-1905; ARC-187 | Approved | Archemix Corp | Zimura, Izervay | United States | Geographic Atrophy; Macular Degeneration | Astellas Pharma Inc, Iveric Bio Inc | 2023-08-04 | Polypoidal choroidal vasculopathy; Wet Macular Degeneration; Geographic Atrophy; Stargardt Disease; Macular Degeneration | Details |
| Zilucoplan Sodium | RA-101495-SC; RA-101495 | Approved | Ra Pharmaceuticals Inc, Ucb Sa | ZILBRYSQ, Zilbrysq | Japan | Myasthenia Gravis | Ucb Japan Co Ltd | 2023-09-25 | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Coronavirus Disease 2019 (COVID-19); Muscular Diseases; Amyotrophic Lateral Sclerosis | Details |
| Crovalimab | RO-711269; SKY-59; RG-6107; RO711269; CH-7092230; RO-7092230; RO-7112689; RO-7112689/F01 | Approved | Chugai Pharmaceutical Co Ltd, F. Hoffmann-La Roche Ltd | 派圣凯, Piasky | Mainland China | Hemoglobinuria, Paroxysmal | Roche Pharma (Schweiz) Ag, Roche (China) Holding Ltd, Genentech Inc | 2024-02-06 | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome; Guillain-Barre Syndrome; Anemia, Sickle Cell | Details |
| Ravulizumab | ALXN-1810; ALXN-1210 | Approved | Alexion Pharmaceuticals Inc, Xencor Inc | Ultomiris | United States | Hemoglobinuria, Paroxysmal | Alexion Pharmaceuticals Inc | 2018-12-21 | Thrombotic Microangiopathies; Kidney Failure, Chronic; Acute Lung Injury; Amyotrophic Lateral Sclerosis; Renal Insufficiency, Chronic; Neuromyelitis Optica; Pneumonia, Viral; Acute Kidney Injury; Atypical Hemolytic Uremic Syndrome; Myasthenia Gravis; Lupus Nephritis; Respiratory Distress Syndrome, Adult; Coronavirus Disease 2019 (COVID-19); Heart Diseases; Kidney Diseases; Hemoglobinuria, Paroxysmal; Dermatomyositis; Glomerulonephritis, IGA | Details |
| Eculizumab | LEX-98; h-5G1.1; 5G1-1; HAL-1 | Approved | Alexion Pharmaceuticals Inc | Soliris, 舒立瑞 | United States | Hemoglobinuria, Paroxysmal | Alexion Pharmaceuticals Inc | 2007-03-16 | Anemia, Hemolytic, Autoimmune; Diabetes Mellitus; Macular Degeneration; Kidney Failure, Chronic; Guillain-Barre Syndrome; Thrombocytopenia; Urea Cycle Disorders, Inborn; Asthma; Nasopharyngeal Carcinoma; Neuromyelitis Optica; Glomerulonephritis, Membranoproliferative; Pre-Eclampsia; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Rejection of organ transplantation; Infant, Newborn, Diseases; Antiphospholipid Syndrome; Delayed Graft Function; Atypical Hemolytic Uremic Syndrome; Hemoglobinuria, Paroxysmal; Vagus Nerve Diseases; Rejection of renal transplantation; End Stage Liver Disease; Myasthenia Gravis; HELLP Syndrome | Details |
| Pozelimab | REGN-3918 | Approved | VEOPOZ | United States | Protein-Losing Enteropathies | Regeneron Pharmaceuticals Inc | 2023-08-18 | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Protein-Losing Enteropathies | Details | |
| Avacincaptad pegol | ARC-1905; ARC-187 | Approved | Archemix Corp | Zimura, Izervay | United States | Geographic Atrophy; Macular Degeneration | Astellas Pharma Inc, Iveric Bio Inc | 2023-08-04 | Polypoidal choroidal vasculopathy; Wet Macular Degeneration; Geographic Atrophy; Stargardt Disease; Macular Degeneration | Details |
| Zilucoplan Sodium | RA-101495-SC; RA-101495 | Approved | Ra Pharmaceuticals Inc, Ucb Sa | ZILBRYSQ, Zilbrysq | Japan | Myasthenia Gravis | Ucb Japan Co Ltd | 2023-09-25 | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Coronavirus Disease 2019 (COVID-19); Muscular Diseases; Amyotrophic Lateral Sclerosis | Details |
| Crovalimab | RO-711269; SKY-59; RG-6107; RO711269; CH-7092230; RO-7092230; RO-7112689; RO-7112689/F01 | Approved | Chugai Pharmaceutical Co Ltd, F. Hoffmann-La Roche Ltd | 派圣凯, Piasky | Mainland China | Hemoglobinuria, Paroxysmal | Roche Pharma (Schweiz) Ag, Roche (China) Holding Ltd, Genentech Inc | 2024-02-06 | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome; Guillain-Barre Syndrome; Anemia, Sickle Cell | Details |
| Ravulizumab | ALXN-1810; ALXN-1210 | Approved | Alexion Pharmaceuticals Inc, Xencor Inc | Ultomiris | United States | Hemoglobinuria, Paroxysmal | Alexion Pharmaceuticals Inc | 2018-12-21 | Thrombotic Microangiopathies; Kidney Failure, Chronic; Acute Lung Injury; Amyotrophic Lateral Sclerosis; Renal Insufficiency, Chronic; Neuromyelitis Optica; Pneumonia, Viral; Acute Kidney Injury; Atypical Hemolytic Uremic Syndrome; Myasthenia Gravis; Lupus Nephritis; Respiratory Distress Syndrome, Adult; Coronavirus Disease 2019 (COVID-19); Heart Diseases; Kidney Diseases; Hemoglobinuria, Paroxysmal; Dermatomyositis; Glomerulonephritis, IGA | Details |
| Eculizumab | LEX-98; h-5G1.1; 5G1-1; HAL-1 | Approved | Alexion Pharmaceuticals Inc | Soliris, 舒立瑞 | United States | Hemoglobinuria, Paroxysmal | Alexion Pharmaceuticals Inc | 2007-03-16 | Anemia, Hemolytic, Autoimmune; Diabetes Mellitus; Macular Degeneration; Kidney Failure, Chronic; Guillain-Barre Syndrome; Thrombocytopenia; Urea Cycle Disorders, Inborn; Asthma; Nasopharyngeal Carcinoma; Neuromyelitis Optica; Glomerulonephritis, Membranoproliferative; Pre-Eclampsia; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Rejection of organ transplantation; Infant, Newborn, Diseases; Antiphospholipid Syndrome; Delayed Graft Function; Atypical Hemolytic Uremic Syndrome; Hemoglobinuria, Paroxysmal; Vagus Nerve Diseases; Rejection of renal transplantation; End Stage Liver Disease; Myasthenia Gravis; HELLP Syndrome | Details |
| Pozelimab | REGN-3918 | Approved | VEOPOZ | United States | Protein-Losing Enteropathies | Regeneron Pharmaceuticals Inc | 2023-08-18 | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Protein-Losing Enteropathies | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Eculizumab biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Hemoglobinuria, Paroxysmal | Details | |
| Eculizumab biosimilar (IBC GENERIUM) | Phase 3 Clinical | Ibc Generium | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details | |
| Gefurulimab | TPP-2511; ALXN-1720; CON-9978 | Phase 3 Clinical | Alexion Pharmaceuticals Inc | Myasthenia Gravis; Proteinuria | Details |
| Eculizumab biosimilar (Biocad) | BCD-148 | Phase 3 Clinical | Biocad | Hemoglobinuria, Paroxysmal | Details |
| Cemdisiran | ALN-62643; ALN-CC5; AD-62643 | Phase 3 Clinical | Alnylam Pharmaceuticals Inc | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Glomerulonephritis, IGA; Atypical Hemolytic Uremic Syndrome | Details |
| Eculizumab biosimilar (Amgen) | ABP-959 | Phase 3 Clinical | Amgen Inc | Hemoglobinuria, Paroxysmal | Details |
| Nomacopan | EV-576; rEV-576; rVA576 | Phase 3 Clinical | Evolutec | Hemoglobinuria, Paroxysmal; Thrombotic Microangiopathies; Pemphigoid, Bullous; Geographic Atrophy; Keratoconjunctivitis | Details |
| Eculizumab biosimilar (Samsung Bioepis) | SB-12; AM004; SB12 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Hemoglobinuria, Paroxysmal | Details |
| Vensobafusp alfa | P014; KP-104 | Phase 2 Clinical | Kira Pharmaceuticals LLC | Hemoglobinuria, Paroxysmal; Glomerulonephritis, IGA; Kidney Diseases; Thrombotic Microangiopathies; Lupus Erythematosus, Systemic; Glomerulonephritis | Details |
| Omoprubart | CAN-106 | Phase 2 Clinical | Beihai Kangcheng (Beijing) Pharmaceutical Technology Co Ltd | Hemoglobinuria, Paroxysmal; Genetic Diseases, Inborn | Details |
| IM-101 | IM-101 | Phase 1 Clinical | ImmunAbs Inc | Autoimmune Diseases | Details |
| ALXN-5500 | ALXN-5500 | Phase 1 Clinical | Alexion Pharmaceuticals Inc | Hemoglobinuria, Paroxysmal | Details |
| RLYB-116 | RLYB-116 | Phase 1 Clinical | Rallybio Llc | Hematologic Diseases; Immune System Diseases; Inflammation | Details |
| NM3086 | NM3086; NM-3086 | Phase 1 Clinical | NovelMed Therapeutics Inc | Hemoglobinuria, Paroxysmal | Details |
| Eculizumab biosimilar (Turgut Ardika) | TUR03; TUR-03 | Phase 1 Clinical | Turgut Ardika Pty Ltd | Details | |
| LP-005 | LP-005; RX-001 | Phase 1 Clinical | Longbio Pharma (Suzhou) Co Ltd | Peripheral Nervous System Diseases; Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Kidney Diseases; Amyotrophic Lateral Sclerosis | Details |
| eculizumab biosimilar(Isu Abxis) | ISU-305 | Phase 1 Clinical | Isu Abxis Co Ltd | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details |
| Eculizumab biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Hemoglobinuria, Paroxysmal | Details | |
| Eculizumab biosimilar (IBC GENERIUM) | Phase 3 Clinical | Ibc Generium | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details | |
| Gefurulimab | TPP-2511; ALXN-1720; CON-9978 | Phase 3 Clinical | Alexion Pharmaceuticals Inc | Myasthenia Gravis; Proteinuria | Details |
| Eculizumab biosimilar (Biocad) | BCD-148 | Phase 3 Clinical | Biocad | Hemoglobinuria, Paroxysmal | Details |
| Cemdisiran | ALN-62643; ALN-CC5; AD-62643 | Phase 3 Clinical | Alnylam Pharmaceuticals Inc | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Glomerulonephritis, IGA; Atypical Hemolytic Uremic Syndrome | Details |
| Eculizumab biosimilar (Amgen) | ABP-959 | Phase 3 Clinical | Amgen Inc | Hemoglobinuria, Paroxysmal | Details |
| Nomacopan | EV-576; rEV-576; rVA576 | Phase 3 Clinical | Evolutec | Hemoglobinuria, Paroxysmal; Thrombotic Microangiopathies; Pemphigoid, Bullous; Geographic Atrophy; Keratoconjunctivitis | Details |
| Eculizumab biosimilar (Samsung Bioepis) | SB-12; AM004; SB12 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Hemoglobinuria, Paroxysmal | Details |
| Vensobafusp alfa | P014; KP-104 | Phase 2 Clinical | Kira Pharmaceuticals LLC | Hemoglobinuria, Paroxysmal; Glomerulonephritis, IGA; Kidney Diseases; Thrombotic Microangiopathies; Lupus Erythematosus, Systemic; Glomerulonephritis | Details |
| Omoprubart | CAN-106 | Phase 2 Clinical | Beihai Kangcheng (Beijing) Pharmaceutical Technology Co Ltd | Hemoglobinuria, Paroxysmal; Genetic Diseases, Inborn | Details |
| IM-101 | IM-101 | Phase 1 Clinical | ImmunAbs Inc | Autoimmune Diseases | Details |
| ALXN-5500 | ALXN-5500 | Phase 1 Clinical | Alexion Pharmaceuticals Inc | Hemoglobinuria, Paroxysmal | Details |
| RLYB-116 | RLYB-116 | Phase 1 Clinical | Rallybio Llc | Hematologic Diseases; Immune System Diseases; Inflammation | Details |
| NM3086 | NM3086; NM-3086 | Phase 1 Clinical | NovelMed Therapeutics Inc | Hemoglobinuria, Paroxysmal | Details |
| Eculizumab biosimilar (Turgut Ardika) | TUR03; TUR-03 | Phase 1 Clinical | Turgut Ardika Pty Ltd | Details | |
| LP-005 | LP-005; RX-001 | Phase 1 Clinical | Longbio Pharma (Suzhou) Co Ltd | Peripheral Nervous System Diseases; Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Kidney Diseases; Amyotrophic Lateral Sclerosis | Details |
| eculizumab biosimilar(Isu Abxis) | ISU-305 | Phase 1 Clinical | Isu Abxis Co Ltd | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details |
This web search service is supported by Google Inc.